Details about Property Measurements:
Thermal Diffusivity:
Transient heat transfer problems occur when temperature distribution changes
with time. The fundamental quantity that enters into heat transfer
situations not at steady-state is the thermal diffusivity. It is related to the
steady-state thermal conductivity through the equation:
D = kpCP
where D is the thermal diffusivity, k is the thermal
conductivity, CP is the specific heat, and p is the material
density. The diffusivity is a
measure of how quickly a body can change its temperature; it
increases with the ability of a body to conduct heat (k) and it decreases with
the amount of heat needed to change the temperature of a body (CP).
| Laser Flash Method:
Thermal properties of materials are established
by experimentally establishing a heat flow boundary value problem, solving the
theoretical equations, and then measuring necessary temperatures or heat fluxes
to determine the thermal property by matching to the theoretical solution. Thus
the easiest theoretical way to measure the thermal conductivity is to set up a
steady state, linear flow of heat through the material and apply Fourier’s
equation. This approach has lead to the development of a number of methods for
measuring thermal conductivity including the Guarded Hot Plate and Linear Rod
methods. These time methods are time consuming and can be susceptible to errors
arising from non-realization of the assumed boundary or steady state conditions.
The flash methods of measuring thermal diffusivity remove the steady state
condition at the expense of measuring the temperature as a varying function of
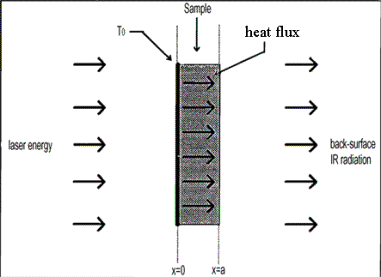
time. With the laser flash method the measurement of the thermal
diffusivity of a material is usually carried out by rapidly heating one side of
a sample and measuring the temperature rise curve on the opposite side (see
schematic at right). The time that it takes for the heat to travel through the
sample and cause the temperature to rise on the rear face can be used to measure
the through-plan diffusivity and calculate the through-plane conductivity if the
specific heat and density are known. |
|
|
|
Holometrix Microflash System:
The method employed by the Microflash system is
as follows. The sample is a disk with a standard diameter of
12.7 mm (~0.5 in) and a thickness ranging from about 0.1 mm to 3 mm (~ 4
– 118 mil). The sample disk is aligned between a neodymium glass laser and an
indium antimonide (InSb) IR detector. The laser is fired several times over a
period of minutes and the necessary data is recorded for each laser “shot”.
The laser beam energy strikes and is absorbed by the bottom surface of the
sample, causing a heat pulse to travel through the sample’s thickness. For a
successful test, the resulting sample temperature rise should be fairly small,
ranging from about 0.5 to 2.0ºC. This temperature rise is kept in the
optimum range by adjusting filters between the laser and the sample. A
lense focuses the back surface image of the sample onto the detector and the
temperature rise signal vs. time is amplified and recorded with a high speed A/D
converter.
|
|

|
Specific
Heat:
The
specific heat of a material is defined as the amount of energy required to raise
a unit mass of material by one unit of temperature at constant pressure.
Cp = Q/m(T2-T1)
where:
Cp
= Specific Heat
M =
mass
T2-T1 = change in temperature
Q =
energy
Specific
heat can be measured with the laser flash method by conparing the temperature
rise of the sample to the temperature rise of a reference sample of known
specific heat tested under the same conditions. This temperature
(voltage) rise is recorded during the diffusivity measurement, so the specific
heat can be calculated from the same data with a suitable calibration.
Assuming that the laser pulse energy and its coupling to the sample remain
essentially unchanged between samples,
Q (absorbed energy) = [mCp(T2-T1)]ref
= [mCp(T2-T1)]sample
and,
Cp sample = [mCp(T2-T1)] / [mCp(T2-T1)]sample
Thermal Conductivity:
The sample thermal conductivity can be calculated with the
diffusivity equation above, after a measurement of the diffusivity and specific
heat has been made and assuming bulk density is known.

